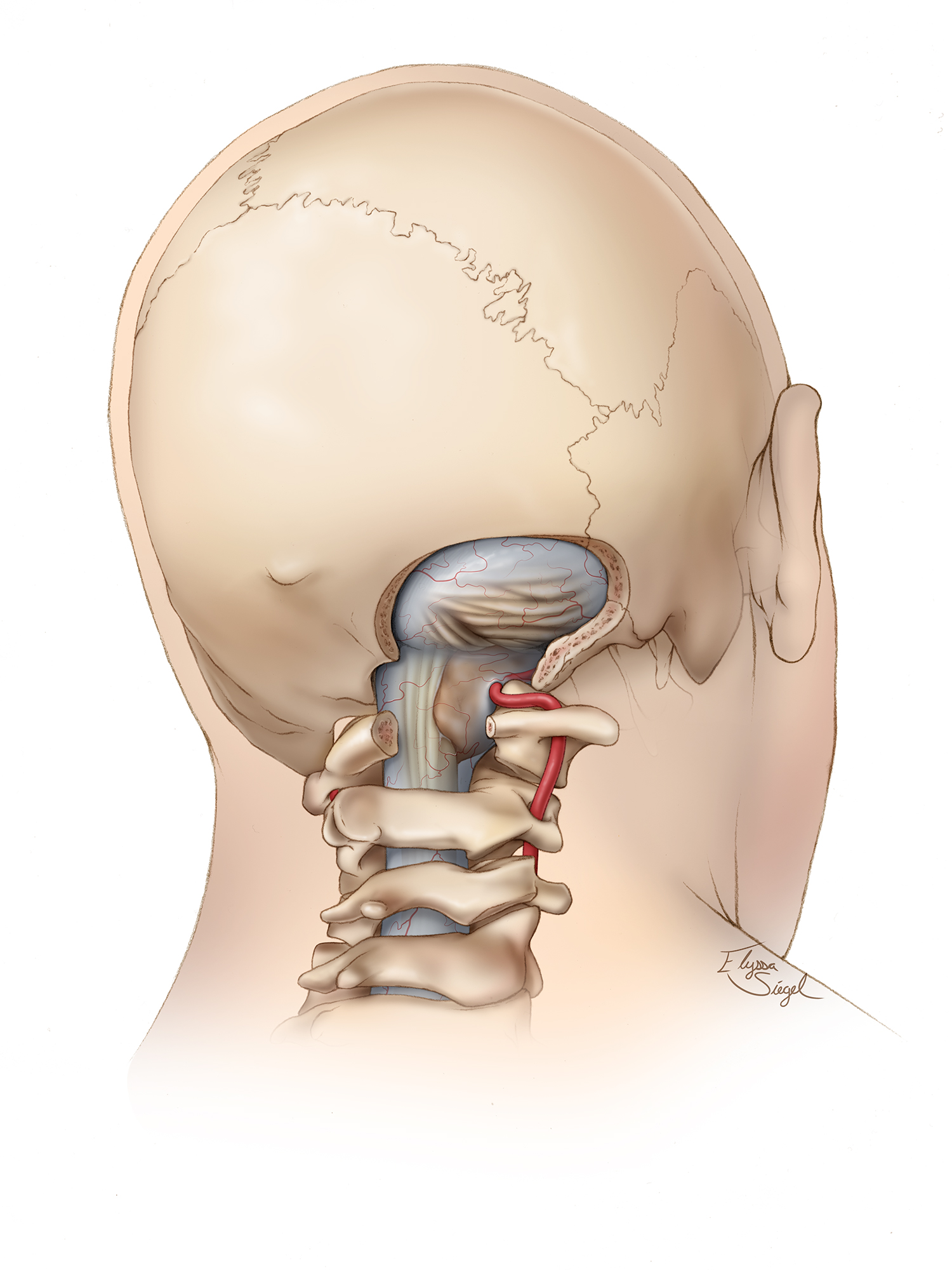
Foramen Magnum Meningioma
This is a preview. Check to see if you have access to the full video. Check access
Resection of a Large Foramen Magnum Meningioma
Meningiomas comprise up to 20% of all intracranial tumors. Although only about 1 to 3% of meningiomas are located at the foramen magnum (FM), this tumor subtype comprises about 75% of all benign, intradural, extramedullary tumors of the cervicomedullary junction.
Like other meningiomas, foramen magnum meningiomas (FMMs) are more frequent in females and rare in children. The neural anatomy around the FM includes the lower cranial nerves, caudal medulla, rostral spinal cord, fourth ventricle, cerebellar tonsils, and inferior vermis. The vascular anatomy includes the vertebral arteries, posterior inferior cerebellar arteries (PICAs), and posterior spinal arteries. Although these structures are vulnerable to mass effect from FMMs, the slow growth rate of the tumor and subtle neurologic findings typically prevent early diagnosis.
Foramen magnum meningiomas originate from the dura of the lower third of the clivus and any portion of the FM circumferentially. Upper cervical spine meningiomas can extend cranially and are commonly called spinocranial FMMs.
For purposes of surgical planning, FMMs are classified in relation to the dentate ligament, which separates the anterior and posterior intradural compartments and the motor and sensory components of the lower cranial nerves (CNs). Most FMMs originate at the anterolateral aspect of the FM, just anterior to the dentate ligament. The second most frequent location is within the posterolateral FM space, followed by purely posterior and anterior FM regions.
More specifically, most FMMs originate just anterior to the dentate ligament, lateral to the entry point of the hypoglossal nerve into its canal, and inferior to the jugular foramen. As a result, the hypoglossal nerve is displaced anteromedially, the medulla is displaced posterolaterally, and CNs IX, X, and XI are displaced superiorly and dorsally and are therefore encountered immediately upon division of the dentate ligament. In these cases, the vertebral artery is typically pinned against the medulla or lateral FM.
Purely anterior lesions originating from the lip of the FM in the midline displace the hypoglossal nerve laterally and may cause the vertebral artery to be pushed in the same direction away from the medulla. Anterior and anterolateral FMMs often create their own surgical corridor through their mass effect on the brainstem, so these tumors are studied preoperatively in relation to the bony anatomy to determine the appropriate extent of bone removal for their exposure.
Posterolateral FMMs arise near the dural entry of the ipsilateral vertebral artery and, as a result, often encase this vessel. The course of the artery in relation to the tumor is identified on preoperative imaging.
Diagnosis
The slow growth of FMMs leads to a prolonged, but often uneventful, neurologic decline. The average time from presentation of symptoms to diagnosis can be as long as 2 to 3 years. The most common early symptom is cervico-occipital pain, elicited with neck flexion and Valsalva maneuvers. Cranial nerve deficits and spastic paresis are usually seen late in the course of the disease.
Lower cranial nerve deficits can be difficult to diagnose because of the compensatory nature of the unaffected contralateral nerve or recruitment of accessory muscles. Hence, patients affected by FMMs may not notice any symptoms or may be only transiently symptomatic.
For example, weakness of the trapezius and sternocleidomastoid muscles, as a result of mass effect on CN XI, can be masked by the recruitment of accessory muscles, leading to muscle atrophy as the only clinical sign.
Progressive spastic quadriparesis may present as foramen magnum syndrome, which is classically defined as unilateral arm sensory and motor deficits that progress to the ipsilateral leg, then to the contralateral leg, and finally to the contralateral arm. Long tract signs characteristic of upper motor lesions manifest as atrophy in the intrinsic muscles of the hands.
Notably, sensory deficits in the territory of the C2 dermatome can occur and should be carefully examined. Symptoms very late in the progression of the disease include intractable pain, motor deficits, ataxia, and the inability to maintain a reliable airway with resultant aspiration pneumonitis.
Figure 1: The tongue should be inspected at rest for atrophy and fasciculations. Note the foramen magnum meningioma in this patient associated with tongue atrophy and fasciculations.
Evaluation
The differential diagnoses for symptoms related to FMMs include cervical spondylosis, multiple sclerosis, amyotrophic lateral sclerosis, syringomyelia, and neuromuscular atrophy disorders. The complexity and timing of symptoms often lead to misdiagnosis of FMMs.
Evaluation of the patient begins with a full history and physical examination, including a detailed examination of cranial nerve and long tract functions. A detailed history often reveals an insidious course of disease followed by a more rapid progression as compensatory mechanisms for neural dysfunction are exhausted.
Weakness of the trapezius and/or sternocleidomastoid muscles may be evident, but pain and atrophy in these muscles is more prevalent. Cranial nerve XII dysfunction is uncommon on presentation; it represents a late finding, and if present, rarely improves after resection.
Long tract signs such as hyperreflexia, Babinski response, and ataxia are evidence of upper motor neuron dysfunction. Swallowing function and speech are formally assessed; their preoperative dysfunction is often worse immediately after surgery, and therefore feeding tube placement is considered soon after surgery to avoid aspiration pneumonia.
Otolaryngologic evaluation assesses vocal cord function. Occult paralysis of the contralateral vocal cord is potentially a contraindication to surgery because ipsilateral iatrogenic CN X injury can result in life-threatening bilateral vocal cord paralysis.
Proper preoperative imaging studies are imperative for diagnosis and surgical planning. Magnetic resonance (MR) imaging sequences of the brain and craniocervical junction are routine. T1 postcontrast sequences are necessary to define the lesion, whereas T2 and fluid attenuated inversion recovery (FLAIR) sequences can delineate the presence of edema within neural structures. Intraparenchymal edema indicates pial invasion and a lack of identifiable intraoperative arachnoid and pial dissection planes for resection.
Furthermore, the bone windows on computed tomography (CT) angiography is useful for evaluating the presence and extent of hyperostosis and the tumor’s relationship to the bony anatomy (particularly the condyles). Calcified tumors are very adherent to the neurovascular structures and usually encase neighboring vessels.
Vascular relationships can be defined with CT angiography (CTA), MR angiography (MRA), or a formal catheter angiogram. CTAs and MRAs are noninvasive methods of evaluating the relationship of the vertebral arteries and posterior inferior cerebellar arteries (PICAs) to the tumor. A formal angiogram allows for real-time evaluation of the flow through the vertebral arteries and the degree of their dominance. Such flow may be compromised due to compression by the tumor.
If the ipsilateral vertebral artery is at risk during resection of a complex tumor, assurance regarding the presence of an intact contralateral vertebral artery for collateral support of flow is paramount. In other words, the contralateral vertebral artery cannot end in the PICA. The extradural origin of PICA (in 5-20% of patients) should be known preoperatively so that it is not injured during dissection at the craniocervical junction.
The dural blood supply involves the posterior and anterior meningeal branches from the vertebral arteries as well as the meningeal branches via ascending pharyngeal and occipital arteries. An angiogram can also help determine if preoperative embolization of the tumor is feasible or helpful.
Figure 2: A classic ventral foramen magnum meningioma with a dural tail along the clivus is demonstrated (top row). This tumor was resected using the conservative transcondylar approach described in the following paragraphs. These tumors can infiltrate the jugular foramen and increase the risk of operative intervention. The presence of brainstem edema indicates pial infiltration by the tumor, requiring subtotal resection for preservation of neurological function (another patient, bottom row).
Indications for Procedure
Treatment options for FMMs include observation with serial imaging, radiosurgery, and surgical resection. The algorithm for rendering treatment is the same as the one for other skull base meningiomas.
Observation is reasonable for small asymptomatic lesions or minimally symptomatic lesions in older patients. Tumors with mass effect in patients who have a reasonable life expectancy should be resected, even if minimally symptomatic, because there is little room within the FM space for continued growth. Radiosurgery has a limited role for large lesions because of the proximity of the brainstem.
Preoperative Considerations
It is necessary to preplan the surgical goals. Tumors that encase the cranial nerves and infiltrate their foramina such as jugular foramen should undergo radical subtotal resection. Intact preoperative lower cranial nerve function should be preserved at the expense of subtotal resection, but thorough brainstem decompression. Radiosurgery can effectively control small residual tumors that are situated away from the brainstem.
Although many tumors seem to encase the vertebral artery on preoperative imaging, reasonable dissection planes can be found intraoperatively. However, vascular stenosis seems to indicate invasion of the vascular wall; in these cases, radical subtotal tumor removal is advised.
I use neurophysiologic monitoring, including somatosensory evoked potentials (SSEPs) monitoring and electromyography of the lower cranial nerves (including CN XII).
Operative Anatomy
The osteology and neurovascular anatomy of the craniocervical junction is complex. For further information, please refer to the chapter on transcondylar approach.
Figure 3: A posterior view of the anterior craniovertebral junction with the brainstem removed is demonstrated. Note the location of the cranial nerves (image courtesy of AL Rhoton, Jr). The most common location for the origin of FMMs is noted (*) on the right side.
Figure 4: A view of the foramen magnum from the posterolateral approach while the vertebral artery is untethered from its dural sleeve is included. Please note the location of CN XI in relation to the adjacent anatomy (image courtesy of AL Rhoton, Jr). The tumor displaces the dentate ligament and CN XI dorsally.
RESECTION OF FORAMEN MAGNUM MENINGIOMA
Foramen magnum meningiomas can be approached from two directions: posterior and posterolateral. Lesions posterior to the dentate ligament are explored through a lateral suboccipital craniotomy with or without laminectomies at C1 and C2. Purely anterior lesions can be approached through endonasal and transoral routes. I reserve the transoral and transnasal routes for extradural lesions of this area and recommend the posterolateral "far lateral" osteotomy for reaching ventral FMMs.
Because FMMs create a surgical corridor by displacing the medulla posteriorly and toward the contralateral side, I prefer a far lateral approach without any condylar resection to accomplish surgical goals for almost all anterior and anterolateral tumors. Tumor remnants have not been due to restricted operative corridors but adherence to neurovascular structures.
Although extensive drilling of the occipital condyle does provide wider exposure, it is unnecessary and risks injury to the hypoglossal nerve. Moreover, removal of more than 50% of the condyle necessitates occipitocervical arthrodesis with a resultant loss of craniocervical mobility and functional impairment. Intracapsular tumor decompression provides additional working space for the surgeon and further negates the need for extensive bone removal. The use of dynamic retraction and strategic operative planning for tumor decompression relieves the need for any fixed retraction on cerebrovascular structures.
Please refer to the Far Lateral Approach chapter for description of the technique.
Figure 5: The most common location for FMMs in relation to the adjacent structures is indicated. The conservative far lateral route provides a reasonable operative trajectory based on the illustrated extent of bone work.
INTRADURAL PROCEDURE
Bone removal is extended laterally to the level of the condyle for ventral and ventrolateral tumors. Following the appropriate osteotomy, the dural entrance of the vertebral artery is evident. Gelfoam powder soaked with thrombin solution or gentle bipolar electrocautery may be used to control bleeding from the vertebral artery’s venous plexus.
Figure 6: The intraoperative photo at the top demonstrates the vertebral artery (VA) at its entry site into the dura, covered by its venous plexus. Minimal condylar resection (*) is performed. The intraoperative photos in the following images correspond to the ventral FMM shown in Figure 2, top row. A curvilinear or hockey stick-shaped dural incision is made (lower image). This method of dural incision permits adequate mobilization of the dura while allowing the operator to create simple edges for watertight approximation at the time of dural closure.
At this stage, it becomes apparent that additional condylar resection may not facilitate more exposure unless the vertebral artery is mobilized out of its bony foramina. In other words, the bulk of the vertebral artery at its dural entrance point will tether the dura and prevent further lateral reflection of the dural flap despite additional condylectomy.
Figure 7: Multiple retraction sutures are placed in the inside surface of the dural flap and close to the bony edges to expand the operative corridor and mobilize the vertebral artery laterally. Once the dura is reflected, C1 and C2 nerve rootlets, along with CN XI (spinal components), should be easily visualized along the tumor’s posterior capsule. The dentate ligament is tented posteriorly. Bone removal should expose the inferior pole of the tumor.
Next, the C1 nerve rootlets are sectioned and CN XI is mobilized laterally. The latter cranial nerve can be adherent to the tumor, and its adhesions are sharply divided. Excitation of the nerve can lead to intermittent movements of the shoulder and surgical target. I often section all rootlets of C1 and one or two spinal rootlets of CN XI to allow adequate exposure of the tumor and mobilization of CN XI. I have not witnessed any significant permanent postoperative shoulder weakness because of this maneuver. The tumor capsule is coagulated.
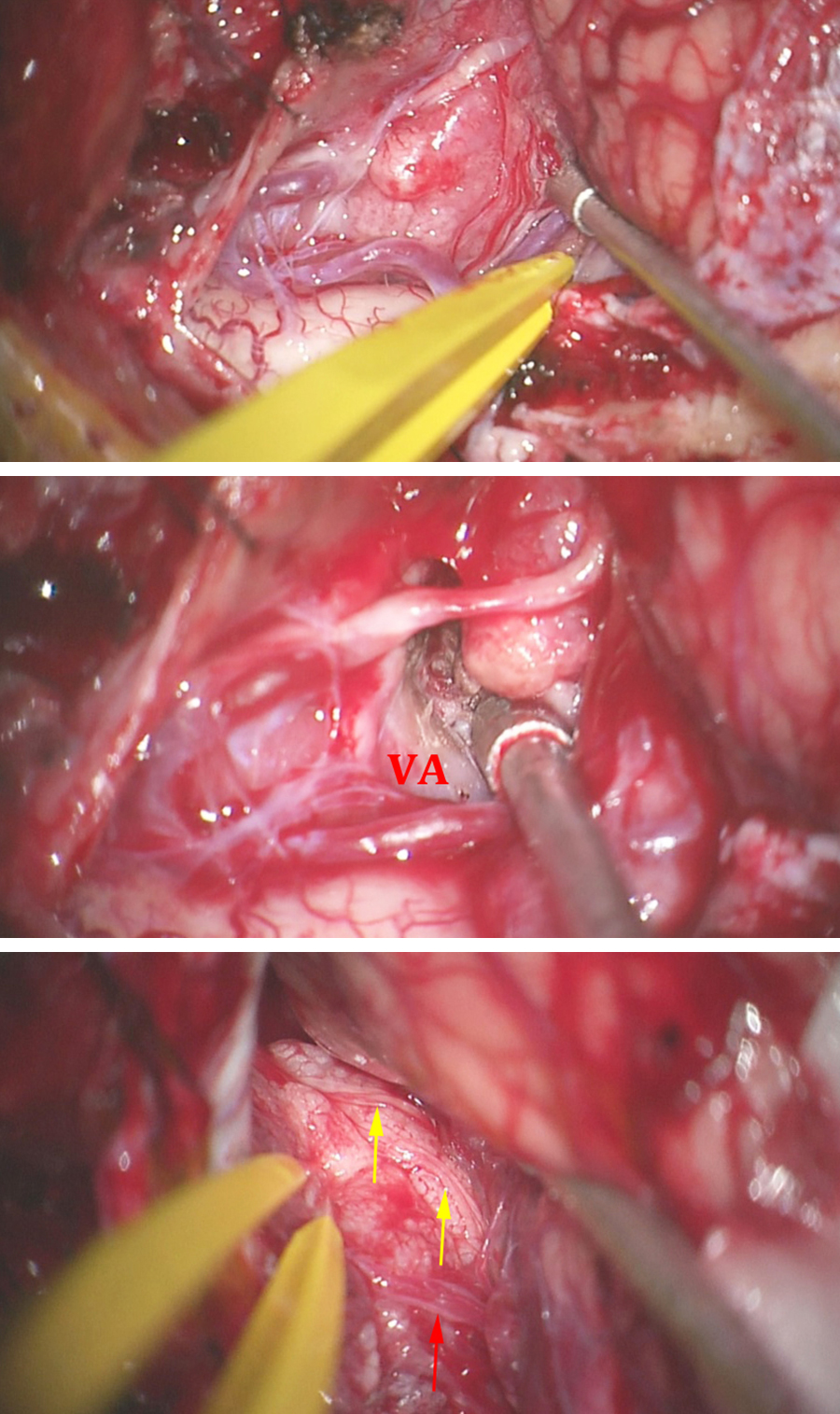
Figure 8: The tumor is first devascularized as much as is safely possible along its lateral attachments, and then centrally debulked using an ultrasonic aspirator. This step is important for decreasing tumor volume and facilitating efficient mobilization of the mass. Debulking of the tumor should be done without violating the medial and lateral poles of the tumor, where neurovascular structures, including the vertebral artery, may reside. The intradural entry point of the vertebral artery is usually easily identifiable. If the artery is encased, doppler ultrasonography may be used for its localization early in dissection. The route of the artery around the tumor can be estimated based on preoperative imaging.
Figure 9: Mobilization of the debulked tumor is an important step in identification and localization of the important neurovascular structures. The vertebral artery and PICA can be adherent to the lateral and superior poles of the tumor capsule or encased within the mass (bottom photo, red arrow). The intradural entry site of the artery is exposed (black arrow). The lower cranial nerves are displaced superiorly and often not adherent to the tumor unless the tumor extends into the jugular foramen (yellow arrow). The vertebral artery may be dissected free and covered with a small cottonoid patty—The use of an ultrasonic aspirator close to the artery can easily lead to its irreversible injury.
Figure 10: Once the tumor is centrally debulked, its capsule can be mobilized laterally and inferiorly. Yellow and back arrows identify the lower CNs and the PICA, respectively, that have been dissected free. Mobilization of the capsule away from the brainstem and spinal cord is done with care, and all of the pial adhesions should be sharply divided and small tumor feeders coagulated. Sharp forceps may be used to punch a hole within the capsule; this maneuver will create a “handle” for the forceps to “drag” and “fold” the tumor capsule laterally. Care must be taken to prevent the tumor capsule from abruptly returning in the medial direction to its original state and injuring the spinal cord.
Figure 11: The suction tip is then placed within the hole in the capsule created by the forceps, holding the tumor away from the medulla and spinal cord. While the suction device clears the operative field by aspirating within the center of the tumor, the ultrasonic aspirator removes the medial tumor capsule.
Figure 12: Finally, additional working space is available via aggressive piecemeal tumor removal. Next, the tumor is dissected along its ventral attachments and the ventral dura is identified. Once the tumor is mobilized away from the neural and vascular structures, it can be removed. The CN XI is protected during various intraoperative movements of the tumor capsule.
The tumor is firmly connected to the ventral and anterolateral FM dura. Tumor mobilization can be difficult, and the operator should avoid uncontrolled forceful tension on the tumor. If tumor mobilization is not easily feasible, careful and meticulous tumor debulking without violating the tumor capsule will ultimately allow lateral mobilization of the medial capsule to allow disconnection of the tumor away from the ventral dura.
Figure 13: Tumor remnants adherent to the ventral dura should be curetted away and coagulated. Resection of the affected ventral dura (X) is usually not safe.
If there are reported changes in the monitored neurophysiological parameters such SSEPs, the force of dynamic retraction is adjusted to relieve any tension on the neural structures. If this maneuver is not effective, blood pressure should be slightly elevated. If the SSEPs remain affected, there is most likely irreversible ischemic insult.
Additional Considerations
Preservation of function is the primary goal of surgery. It may be necessary to leave behind small pieces of tumor adherent to the vertebral artery, brainstem, and spinal cord pia. If the tumor is infiltrating into the jugular foramen, the lower CNs are encased by the tumor and subtotal resection is advised. Dissection of the functioning lower CNs within the tumor is not safe.
Figure 15: A left-sided fibrous FMM is demonstrated. Note the tumor was engulfing the vertebral artery; the CTA demonstrates the route of artery. The approach is demonstrated in the following images of this series. The extent of minimal condylectomy is marked with a yellow arrow and the dural entrance of the artery is noted with a red arrow.
Figure 16: The tumor was unveiled (upper image) and the vertebral artery was localized within the center of the tumor. The CN XI was engulfed within the superior pole of the mass (middle image). The lower cranial nerves (yellow arrows) were also surrounded by the mass (lower photo), precluding any chance at gross total tumor removal. A perforating artery of the PICA (red arrow) was also surrounded by the tumor.
Figure 17: I decompressed the brainstem via skeletonization of the vertebral artery. The superior pole of the tumor was left untouched to protect the CNs and perforating vessels. The inferior dural attachments were coagulated.
Subtotal Resection of an Encasing Foramen Magnum Meningioma via the Far Lateral Approach
Closure
Watertight dural closure is necessary. The bony defect is usually small and cranioplasty is not routinely performed.
Postoperative Considerations
The patient is closely monitored in the intensive care unit after surgery. Swallowing function is formally evaluated next day before feedings are resumed. Patients with preoperative lower cranial nerve dysfunction should remain intubated until otolaryngological consultation has been obtained and their cough and gag reflexes are formally assessed.
Patients who fail their extubation trials or swallowing evaluations more than twice will undergo tracheostomy and gastrostomy procedures, respectively. Temporary shoulder weakness is common due to intraoperative manipulation of CN XI.
Pearls and Pitfalls
- Far lateral suboccipital craniotomy and C1 laminectomy along with minimal condylectomy are adequate for resection of anterolateral and anterior FMMs.
- “Far lateral” or “extreme lateral” approaches with vertebral artery mobilization are, in my opinion, unnecessary for resection of ventrolateral and purely ventral tumors.
- Aggressive tumor debulking is critical for safe tumor mobilization and its excision while avoiding traction on the surrounding vital cerebrovascular structures.
- If pial or foraminal invasion by the tumor is encountered, a thin sheet of tumor must be left behind to avoid deleterious postoperative deficits.
Contributor: Andrew R. Conger, MD, MS
For additional illustrations of the far lateral approach to the foramen magnum, please refer to the Jackler Atlas by clicking on the image below:
References
Al-Mefty O. Operative Atlas of Meningiomas. Philadelphia: Lippincott-Raven, 1998.
Borba LAB, de Oliveira JG, Giudicissi-Filho M, Colli BO. Surgical management of foramen magnum meningiomas. Neurosurg Rev. 2009;32:49–58; discussion 59–60.
Flores BC, Boudreaux BP, Klinger DR, Mickey BE, Barnett SL. The far-lateral approach for foramen meningiomas. Neurosurg Focus. 2013; 35:E12.
Please login to post a comment.