Lumbar Drain Free
This is a preview. Check to see if you have access to the full video. Check access
Placement of Lumbar Drain
Lumbar drainage of cerebrospinal fluid (CSF) has many applications in neurosurgery. It can be used for decompression of CSF spaces, management of intracranial hypertension, and repair of a CSF leak or fistula. Clinical indications, successful insertion, and postoperative management are important skills that will be reviewed here.
One-time lumbar puncture may be performed as a diagnostic measure or when large volume CSF drainage is unnecessary. For example, I perform a lumbar puncture immediately before a retromastoid craniotomy. This maneuver allows drainage of ~35 cc of CSF and provides a smooth and unobstructed reach toward the cerebellopontine angle cisterns and around the relaxed hemisphere, with minimal retraction on the cerebellum. In general, I have a low threshold for using intraoperative lumbar drainage to achieve brain relaxation.
Indications for the Procedure and Complication Avoidance
Obstructive hydrocephalus is a contraindication for the use of a lumbar drain, but in my opinion, tumor size and moderate midline shift are not prohibitive as long as CSF spaces are communicating. A lumbar drain can be placed prior to craniotomy in select patients to provide brain decompression. This is especially important for procedures when the CSF cisterns are not accessed early during the intra- or extradural procedure and immediate brain mobilization is necessary or when the tumor has partially filled the CSF cisterns approached through the operative corridor. These procedures include:
- subtemporal approaches, including extradural middle fossa osteotomy and anterior petrosectomy;
- interhemispheric dissection without early access to the ventricle;
- extradural clinoidectomy;
- supracerebellar trajectories;
- bifrontal basal approaches; and
- retromastoid and subfrontal trajectories with evidence of increased intracranial tension.
I use lumbar drainage liberally for most deeply located meningiomas, especially when I cannot readily access the CSF cisterns (i.e., for parafalcine and large parasagittal meningiomas). Similarly, this form of CSF drainage is effective for resection of tumors filling the basal cisterns (i.e., for large clinoidal and tuberculum sella meningiomas). The initial brain decompression allows easy access to the base of the tumor for its early devascularization without a need for aggressive brain retraction or transgression. This devascularization dramatically enhances operative efficiency through hemostasis during tumor removal and dissection.
In large tumors with significant mass effect, I open the lumbar drain during the dural opening and avoid CSF egress before then to minimize the risk of herniation syndromes. Tumors that cause a significant edema often lead to brain herniation through the craniotomy defect during and after dural opening, and lumbar CSF drainage can prevent cortical contusions caused by this herniation.
Although I avoid large-volume CSF egress during insertion of the lumbar drain, CSF may be removed in 10-20 cc aliquots for a maximum total of 60 cc during the dural opening and the entire procedure to achieve the desirable brain relaxation. If CSF cisterns are reached, the lumbar drain is clamped for the reminder of the operation to avoid symptomatic pneumocephalus. Unless a risk of CSF fistula is present because the air sinuses have been violated, the lumbar drain is removed at the end of the surgery.
There are certain considerations that are worth further emphasis. I use a lumbar drain to divert CSF for ~48 hours after surgery for skull base procedures that may lead to large dural defects joining nasal sinuses (expanded endonasal and bifrontal transcribriform and transtubercular osteotomies). There are rare patients who have no evidence of a leak immediately postoperatively, but who demonstrate a significant amount of nonresolving pneumocephalus on their postoperative CT scan. In this situation, a large leak must be suspected and corrected through a repeat operation. Otherwise, continuation of lumbar drainage will lead to “reverse suctioning” of air through the nose and dramatic worsening of the pneumocephalus. These cascades can explain an acute neurologic deterioration and infection. A repeat operation with careful dural closure and skull base reconstruction using vascularized regional and free tissue transfer flaps is often necessary.
I avoid simultaneous drainage through an external ventricular drain and lumbar drain as this may lead to dramatic changes in CSF dynamics. These alterations can potentially cause acute development of a large amount of pneumocephalus associated with neurologic deterioration.
I have fortunately never witnessed transtentorial herniation caused by intraoperative lumbar CSF drainage. Nor have I seen postoperative Chiari or symptomatic intracranial hypotension and cerebral “settling.” However, some colleagues have reported these complications occurring with overzealous lumbar drainage.
Details of the Procedure
Placement of a lumbar drainage system is simple but can lead to unexpected complications.
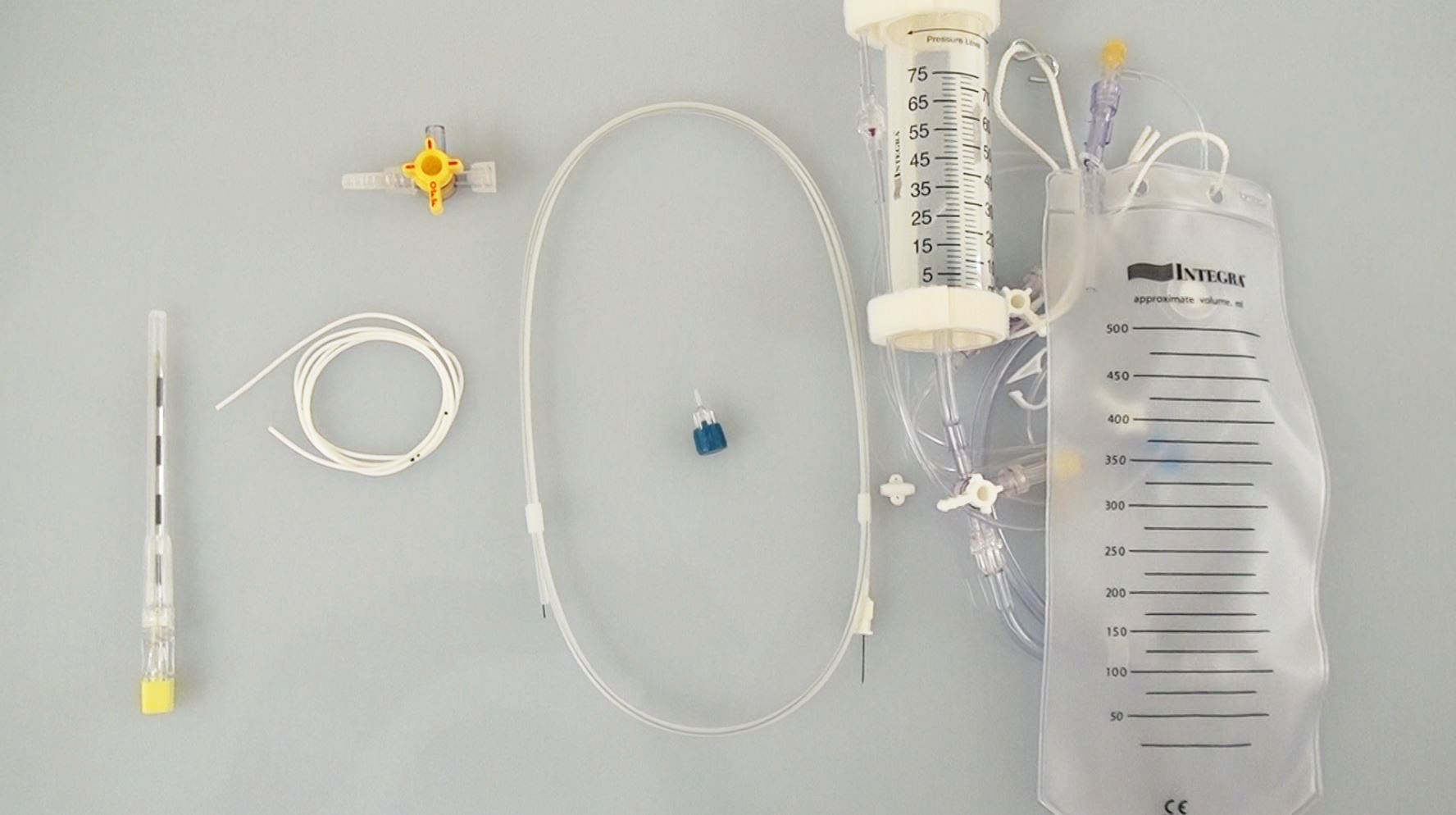
Figure 1: The components of the system necessary for placement of a lumbar drain include a 14-gauge Tuohy needle.
Figure 2: The patient is placed in the lateral decubitus position. Although not shown in this image, the fetal position expands the interlaminar space and facilitates needle insertion and cannulation of the thecal sac. The iliac crest is palpated to estimate the position of the L4-5 interspace. The patient’s torso should not be rotated to avoid confusion regarding the location of the midline in the coronal plane.
Figure 3: The Tuohy needle is inserted at the midline and through the interspinous space with the beveled side facing cephalad. I often advance the needle in a slightly cephalad direction and follow the contours of the adjacent spinous process. If I come in contact with the lamina, I “walk” the needle tip along this bone with fine adjustments until the needle “falls into” the interlaminar space. Alternatively, the paraspinal technique may be used to angle the needle from lateral to medial to reach the sac.
Difficulty in cannulating the thecal sac can be because:
- The interlaminar space is narrow and the spinous processes deflect the needle tip away from its desired trajectory without the knowledge of the operator who is only manipulating the distal end of the needle. Any small deflection or error in the needle trajectory can prevent the needle from entering the interlaminar space. Degenerative disorders in older patients cause this narrowing and complicate efforts to reach the thecal sac.
- The needle tip is pointing away from the midline; the operator is disoriented regarding the exact location of the midline. If the patient is in the lateral decubitus position, a slight unintended rotation of the torso can lead to this confusion.
- In morbidly obese patients, a longer needle may be necessary to reach the thecal sac.
- The thecal sac is decompressed and collapsed due to previous attempts and resultant CSF drainage and cannot be reliably entered with the needle tip.
The surgeon can detect changes in needle resistance after the needle traverses through the interlaminar ligament.
Figure 4: Once the thecal sac is penetrated and CSF is obtained, the orientation of the Tuohy needle is turned so that he beveled side of its tip is pointing cranially. The lumbar drain catheter can now be threaded over a wire and tunneled through the Tuohy needle after removal of the needle’s stylet. I often first attempt threading the catheter without the wire, and if I encounter resistance, I then use the catheter over the wire.
If the catheter cannot be advanced through the theca sac, the following are likely the reasons:
- the needle tip may be too deep and should be slightly withdrawn; the catheter’s progress is prevented by the posterior vertebral body, or
- alternatively, the angle of the needle is not ideal and the needle trajectory should be adjusted by its withdrawal and repositioning.
Typically the catheter is advanced through the needle to the level of the T12-L1 (4-5 cm beyond the needle tip). The wire and the needle are then removed and the catheter is secured with tape. A sterile occlusive dressing is placed and the catheter is attached to the lumbar drainage bag.
Figure 5: I usually place two pieces of sponge to straddle the catheter at its exit point on the skin to prevent its kinking. The sponges are held in position using a piece of Tegaderm. If the drainage system stops working when the patient is in the supine position, the catheter is most likely kinked near the skin exit and this area should be padded.
Alternative Methods
If the catheter can not be readily threaded into the thecal sac, other options may be available.
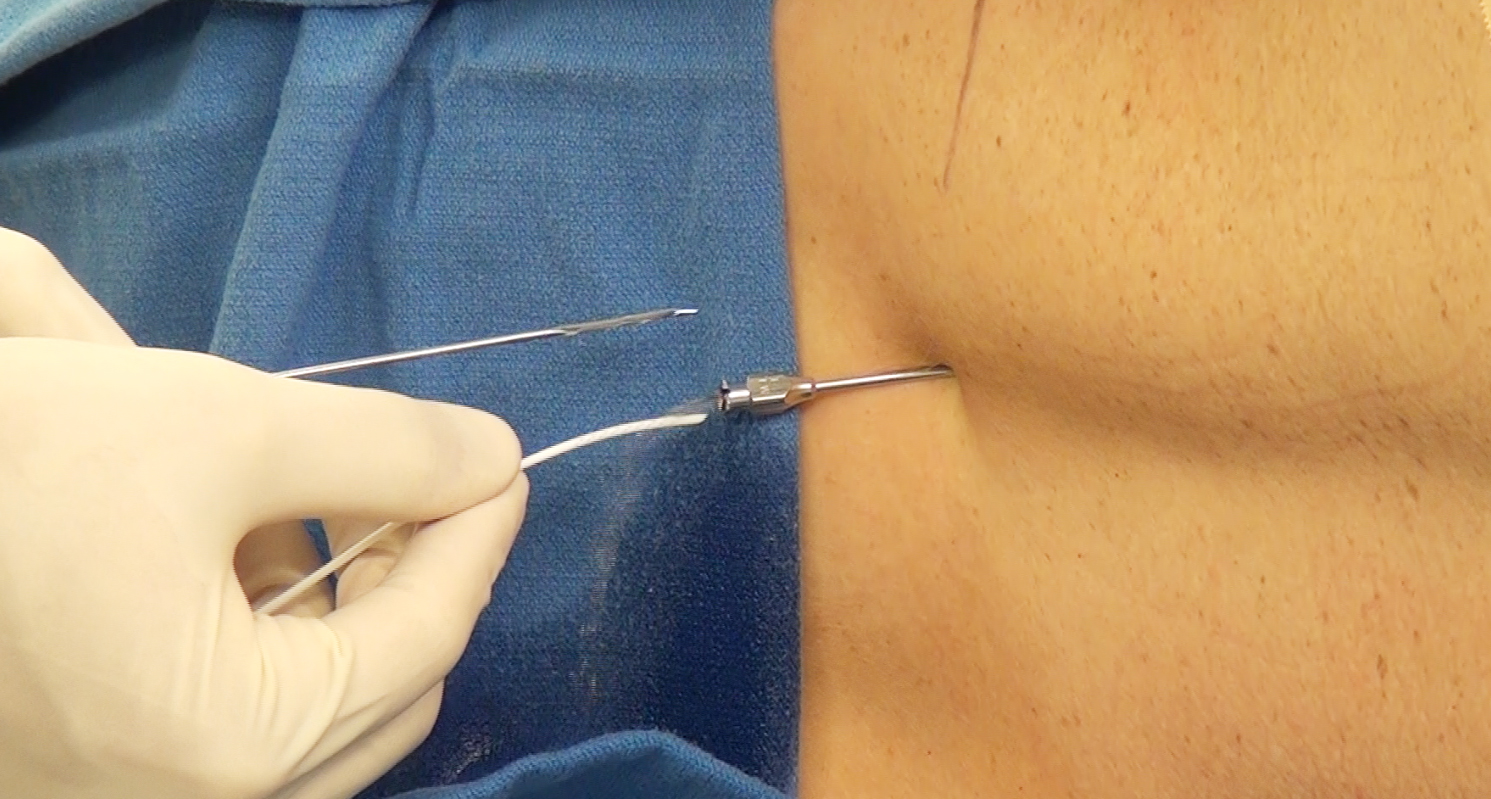
Figure 6: Alternative methods of lumbar drainage include insertion of only the needle and its fixation on the skin (top image). In this model, the catheter is not used. An IV tubing is directly connected to the needle hub. This maneuver is possible for patients who are placed in the lateral position during surgery. It is important to place large blankets to straddle the needle so the needle is not inadvertently displaced during the procedure (lower image).
Intraoperative Lumbar Drain: Leaving Needle in
Drain Management
Proper management of the lumbar drain is important to avoid under- and overdrainage complications. CSF can be drained every hour at a specific volume or to a specific pressure. To manage by volume, the surgeon can choose an hourly volume of drainage (i.e., 8–15 cc per hour). The drain can be intermittently clamped to maintain this level.
Alternatively, the drain can be placed at a specific level and CSF will drain when the patient’s CSF pressure exceeds the leveled pressure near the tragus or shoulder. To avoid overdrainage, the lumbar drain should be clamped when the patient is out of bed and leveling should be adjusted based on the patient’s position. This model is complicated, labor intensive, and can be problematic for the nursing staff.
Underdrainage and overdrainage can both lead to morbidity or even mortality and their early recognition can be life-saving. The corresponding clinical symptoms should be recognized immediately and appropriate action taken. Serious complications from lumbar drainage include tension pneumocephalus, transtentorial herniation, and subdural or intracerebral hematoma formation. These events may lead to acute neurologic deterioration.
I have left lumbar drains in place for as long as 10 days with no untoward effect. I do continue antibiotics while the drain is in place. If the drainage tubing is disconnected while the patient is on the floor, I clean the disconnected ends with Betadine solution and reconnect them; I do not replace the entire drainage system.
Pearls and Pitfalls
- Lumbar CSF drainage can provide a great opportunity for cerebral decompression and avoidance of retraction injury during skull base and interhemispheric procedures.
- Appropriate lumbar drain management is important because life-threatening complications can occur from over- or under-drainage.
Please login to post a comment.