Spinal Cord AVM
This is a preview. Check to see if you have access to the full video. Check access
Spinal Cord Arteriovenous Malformation: Techniques for Resection
Please note the relevant information for patients suffering from arteriovenous malformations is presented in another chapter. Please click here for patient-related content.
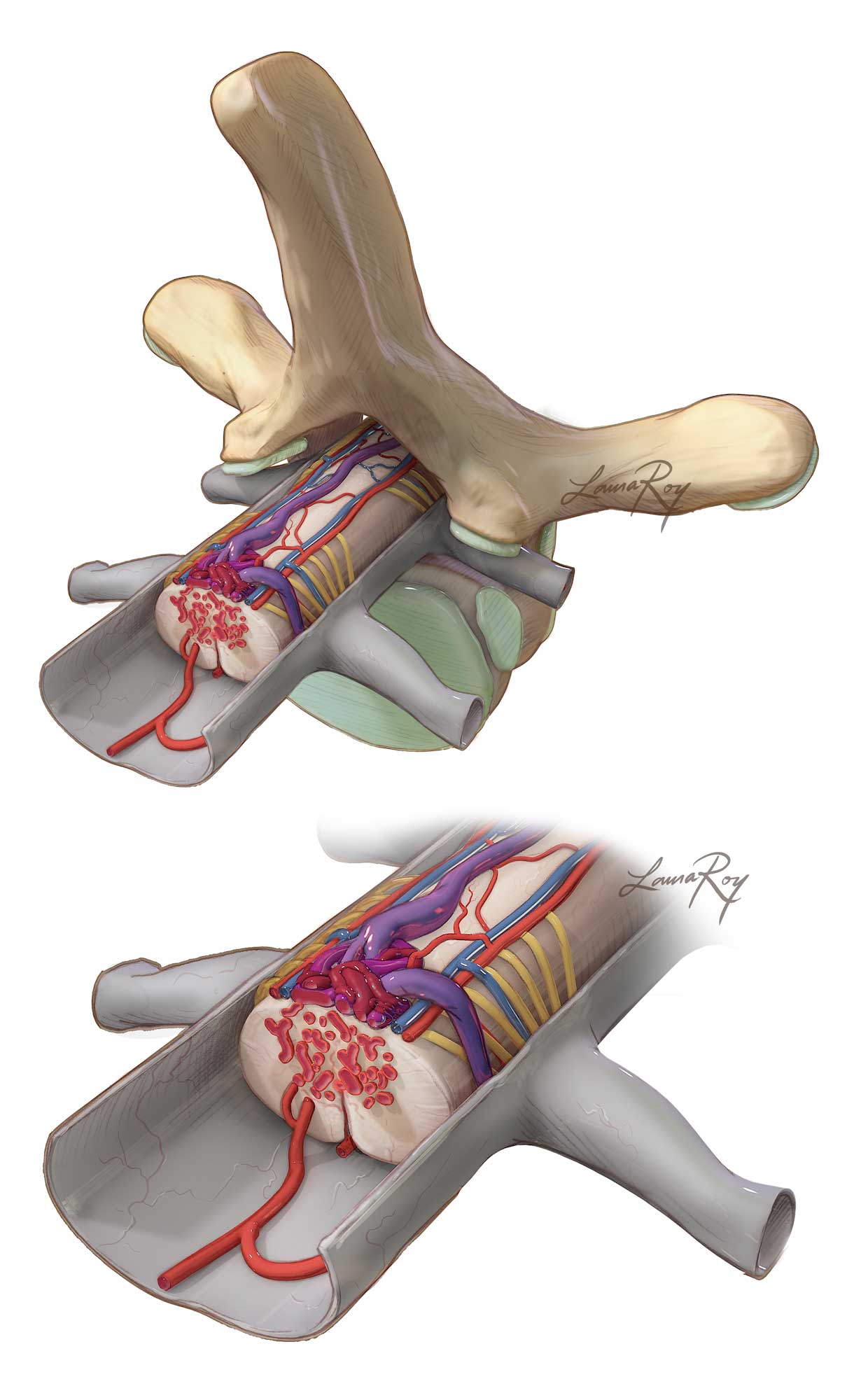
Spinal cord arteriovenous malformations (AVMs) or glomus AVMs are true intraparenchymal vascular lesions. They are characterized by a short compact nidus within the substance of the cord, which sometimes extends to the extramedullary compartment of the spinal canal.
Spinal AVMs are rarer than spinal dural fistulas and are a minority of all spinal vascular malformations. They are neuroanatomically analogous to intracranial AVMs, but differ in certain features. Importantly, their subtotal resection via the pial resection technique (see below) is well tolerated, unlike their intracranial counterparts.
Their occurrence in younger patients, their association with other vascular anomalies, and their uniform distribution along the spinal cord, in comparison with spinal dural arteriovenous fistulas, indicate a congenital origin of spinal AVMs as a result of inborn errors during vascular embryogenesis.
Spinal AVMs are typically supplied by at least one radiculomedullary artery arising from the anterior or posterior spinal arteries that feeds the spinal cord and the AVM. They are classified as type II spinal vascular malformations according to the modern classification system. For a detailed discussion on classification of spinal vascular malformations, please refer to the Spinal AV Fistula Disconnection chapter. For relevant vascular anatomy of the spinal cord, please refer to the Operative Spinal Cord Anatomy chapter.
In this chapter, I will discuss the presentation, management, and surgical nuances for resection of true spinal cord AVMs.
Clinical Presentation
Spinal AVMs typically affect patients in their third decade of life, have no gender predilection, and occur mostly in the thoracic spinal cord (more than half of the cases). The second most common site is the cervical spine (~30%). There is a high incidence of associated arterial aneurysms and 13% to 37% occurrence of other central nervous system vascular abnormalities.
Type II lesions are true high-flow AVMs. Analogous to spinal fistulas, four major pathophysiological mechanisms are involved in the pathogenesis of spinal AVMs: 1) venous hypertension, 2) vascular steal syndrome, 3) hemorrhage (subarachnoid or intraparenchymal), and 4) mass effect. They give rise to a very distinct bimodal clinical presentation, namely acute deterioration or progressive neurologic decline.
Acute deterioration is much more frequent than with spinal fistulas. Sudden onset or ictal back pain, meningismus, suboccipital pain, and/or sudden loss of consciousness are the usual complaints by 35% to 86% of patients, thereby reflecting subarachnoid or intraparenchymal hemorrhage. At the time of diagnosis, about half of the patients have experienced one or more episodes of hemorrhage. This is especially true in children, among whom 84% present with an acute onset followed by 59% with sudden impairment of motor function.
Massive hemorrhage may be followed or not by neurologic deficits, but the occurrence of excruciating back pain is also referred to as the le coup de poignard rachidien, or coup de poignard of Michon, as an illusion of being stabbed in the spine.
The annual risk of hemorrhage from spinal AVM is estimated at 4%, increasing to 10% for patients with previous hemorrhage. Similar to intracranial AVMs, a previous bleeding episode and the occurrence of an associated aneurysm (in the feeding artery or intranidal in up to 48%) increase the risk of hemorrhage by twofold.
The remainder of patients with no hemorrhagic presentation suffer from progressive neurologic decline as a result of myelopathy caused by vascular steal, venous hypertension, or mechanical compression by an aneurysm. Patients commonly suffer from a different combination of paresis/paralysis (65-75%), paresthesias (40-60%), pain (10-51%), and bladder/bowel dysfunction (15-42%). Unlike spinal fistulas, symptoms are not affected by exercise or posture. Conus medullaris AVMs usually present with radicular symptoms (~60%) and have a higher incidence of sphincter dysfunction.
The differential diagnosis for the above-mentioned symptoms and signs is extensive, and the surgeon should consider other etiologies that present in a similar manner, including spinal cord tumors or demyelinating conditions. A spinal dural fistula can cause similar signs, but the patient’s age at presentation is a reliable distinguishing factor between these two lesions, with AVMs occurring in patients 15 to 40 years old.
Figure 1: A schematic representation of the typical angioarchitecture of spinal AVMs (type II) is illustrated. The presence of en passage arteries to the spinal cord complicates the resection risk of these daunting lesions.
Diagnosis and Evaluation
As with other spinal vascular lesions, a high index of suspicion is a key issue for diagnosis, especially after excluding degenerative, tumorous, and demyelinating entities.
On physical examination, two rare signs are extremely useful when present, namely a spinal bruit and/or a cutaneous angioma. Definitive diagnosis is confirmed by imaging.
Magnetic resonance (MR) imaging is the first imaging study performed during the investigation of spinal vascular malformations. Spinal AVMs typically show focal enlargement of the spinal cord and flow voids with a serpentine pattern on T1- and T2-weighted images. Subacute hemorrhage shows as a high signal on T1-weighted sequences. Analogous to spinal fistulas, venous congestion may be present. Globular-shaped flow voids indicate aneurysms or venous varices.
The nidus differentiates an AVM from a perimedullary arteriovenous fistula. MR findings are useful for diagnosis, but correct classification of the spinal vascular malformation may have to wait for angiography. New MR angiography (MRA) protocols, such as fast contrast-enhanced, and CT angiography, are often able to precisely determine the level of the fistulous connection, but are not able to supplant spinal angiography.
Digital subtraction spinal angiography allows the clinician to confirm the diagnosis and study the angioarchitecture of the spinal AVM. This modality is important for precise localization of the pathology and surgical or endovascular planning. The study must accurately identify all of the contributing arterial feeding vessels, the nidus, and the draining venous system. A dense mass of agglomerated vessels over a short segment of the spinal cord is frequently encountered. The presence of feeding and/or intranidal aneurysms or venous stenosis affects the natural history of these lesions.
Figure 2: A 48-year-old woman presented with progressive left-sided weakness and numbness. Thoracic MR imaging disclosed hypervascularity (serpiginous flow voids) within the dorsal intradural space together with large intraparenchymal flow voids at T8-9, suggestive of a nidus. Spinal cord edema (left image) was noted. On the axial image (right image), this spinal AVM appeared to have both intramedullary and extramedullary components on the left side.
Figure 3: Selective catheter angiography (early and late arterial phases) identified a compact glomus malformation, which was fed by radiculomedullary arteries near T9.
Treatment Paradigms
The treatment of spinal AVMs is multidisciplinary and involves microsurgery (with or without embolization) and embolization alone. The ideal treatment would be the one that provides complete and durable spinal AVM obliteration while preserving the vascular supply of the spinal cord.
The decision-making process for selection of the final mode of therapy requires a consideration of the lesional angioarchitecture and its extent of cord involvement as well as the surgical and endovascular expertise available at the treating facility.
For proper patient selection, the risks of treatment should not outweigh the risks involved in the AVMs’ natural history. Some spinal AVMs may need to be followed conservatively. In certain situations, however, a less than definitive treatment is preferred in order to minimize symptoms and preserve neurologic function. Partial obliteration over multiple embolization treatments may relieve symptoms and minimize the risk of injury to the spinal cord.
Large size, thoracolumbar location, ventral position within the spinal cord, complex blood supply, multiple feeders from the anterior spinal artery, prenidal aneurysms, and large venous varices are some of the reported conditions associated with an increased risk of permanent morbidity, regardless of the therapeutic method chosen.
Surgery is usually performed in a delayed fashion in order to permit clot lysis and reabsorption after subarachnoid or intraparechymal hemorrhage.
The overall rates of complete obliteration are approximately 80% with microsurgical treatment and 35% with endovascular therapy alone. Long-term morbidity is rather similar between both treatment modalities (~10%). Some colleagues have reported that complete obliteration and partial embolization are associated with a significant reduction in hemorrhage rate. Partial surgical treatment has been reported to carry an annual bleeding risk of 3%.
In select cases, early intervention before the development of disabling symptoms is associated with good neurologic recovery. Surgical short-term results (3 years of follow-up) indicate that 60% to 100% of patients improve or at least maintain their preoperative clinical status.
Alternative Approaches
Endovascular therapy has been used more frequently in the Onyx era and has been indicated as the treatment of choice for spinal AVMs by several authors in recent literature. Particulate embolization may be recommended before surgery to reduce AVM size, but the incidence of recanalization is quite high.
Endovascular embolization should be attempted with liquid embolisates at the nidus, thereby avoiding the occlusion of proximal or distal vessels that supply the spinal cord. Endovascular management has limited use on small tortuous vessels and when feeding arteries share their arterial pedicle with the anterior spinal artery.
The results of embolization appear promising, and further evaluation with long-term results is still required to define its role in spinal AVM management.
Radiosurgery has been reported anecdotally in the literature, but since it has not been extensively studied, it is not recommended routinely in the management of spinal AVMs.
Microsurgery
Surgical management is reasonable for the following lesions:
- lesions in a dorsal location, and
- spinal AVMs that are partly extramedullary (exophytic).
Preoperative Considerations
After diagnostic imaging, patients are classified according to their functional capabilities. The main scoring system for spinal AVMs is the Aminoff-Logue disability scale, which can be found in the Spinal AV Fistula Disconnection chapter.
I do not routinely use preoperative embolization for most AVMs because the risk of embolization is significant, especially for spinal AVMs, and the benefits are modest at best. Large feeding pedicles that are amenable to embolization are readily accessible during surgery.
Embolization methods also have limited use when arteries are tortuous, small, and numerous and when the feeder arteries share their origin with major contributors to the anterior spinal artery or the artery of Adamkiewicz. In these cases, provocative testing via the infusion of amytal sodium may be performed.
Nonetheless, embolization proves particularly effective for reducing the blood supply from anteriorly located feeding arteries that are not reachable from posterior or posterolateral approaches.
MICROSURGICAL SPINAL AVM RESECTION
For a detailed discussion on perioperative patient care, surgical positioning, and surgical approach, please refer to the Extramedullary Spinal Cord Tumor chapter.
For spinal AVMs, a posterior midline approach and its expanded modifications are all that are needed for safe exposure. I prefer a wide laminectomy while preserving the capsule of the facet joint. I extend the laminectomy one level above and below the lesion to provide adequate exposure. Partial medial facetectomy or costotransversectomy is usually required for the management of ventrally placed AVMs.
Immaculate epidural hemostasis using gelfoam powder soaked in thrombin is paramount to achieve uninterrupted pristine microsurgery after dural opening.
I open the dura in the midline while attempting to preserve the arachnoid until the entire extent of the AVM can be estimated through it. I then incise the arachnoid with the utmost care using microscissors or an arachnoid knife to preserve all adherent vessels.
Dorsal, dorsolateral, and ventrolateral lesions are easily identified after the arachnoid opening. Purely intramedullary lesions demonstrate hemosiderin deposits on the pial surface.
Anterior or anterolateral lesions require a slightly paramedian durotomy. Some of the denticulate ligaments and select dorsal nerve rootlets (particularly in the thoracic spine) are sacrificed and gently rotated in order to increase the anterolateral surgical trajectory.
Intraoperative indocyanine green (ICG) angiography may be necessary to localize the malformation and reliably distinguish afferent from efferent vessels. I liberally use this technique to better understand the angioarchitecture of the malformation and to define the early feeding vessels before pedicle sacrifice. The draining veins should be protected until the end of the procedure. The en passage vessels are strictly preserved.
Embolization material helps me identify feeding arteries, also allowing me to correlate the surgical anatomy with the AVM angioarchitecture on the preoperative images.
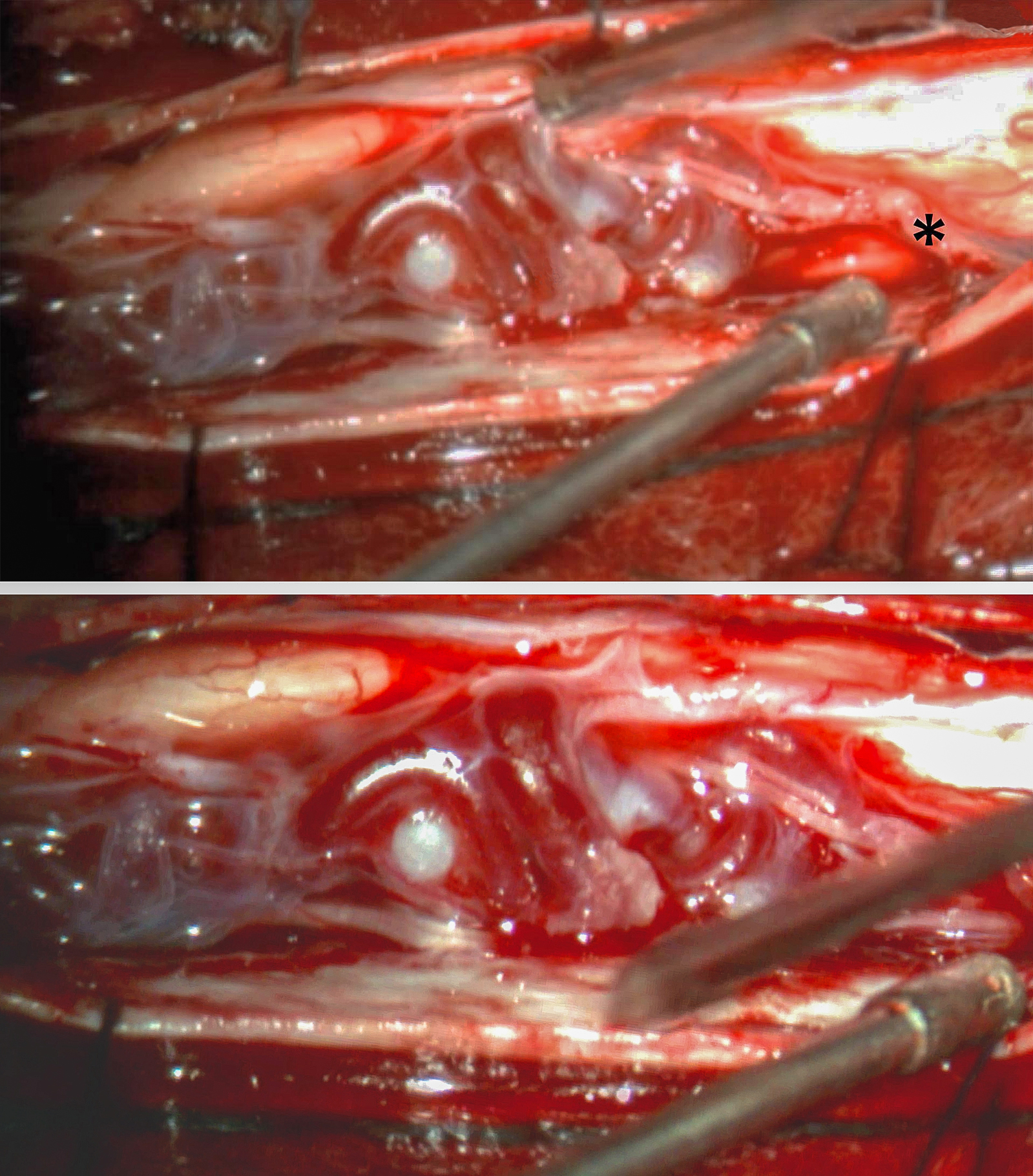
Figure 4: The initial inspection after arachnoid opening reveals a laterally positioned glomus malformation. A major feeder was readily identified along the inferior aspect of the malformation (upper photo, asterisk). See Figures 2 and 3 for preoperative images. A more magnified view of the exophytic part of the malformation is also shown (lower photo).
Figure 5: Spinal AVMs are approached from the arterial side initially. Large feeders are clip ligated with standard short aneurysm clips (asterisks) and subsequently dissected, coagulated under continuous irrigation, and transected. Next, the clips are removed so the dura can be closed in a watertight manner at the end of the operation. For smaller AVMs, however, metallic clips can be very cumbersome and restrictive for microsurgery. Intraoperative electrophysiologic monitoring via somatosensory and motor evoked potentials is routinely performed throughout surgery. It is especially useful during microclip temporary occlusion because any deterioration should result in clip removal to avoid cord injury. The feeder should then be carefully reinspected and its en passage branches protected.
Figure 6: Resection of lesions with significant intramedullary extension may carry a higher risk of postoperative morbidity. In such instances, the pial resection technique is advocated, via which the feeding arteries and draining veins are coagulated over the pial surface and the exophytic portion of the AVM is resected, but the intramedullary component left intact. This maneuver minimizes subpial preparation and the risk of permanent morbidity. The gliotic plane is respected. This technique provides subtotal nidal resection by cutting across the malformation, but it has proven highly effective during spinal AVM’s devascularization.
Figure 7: In this schematic representation, the pial resection technique is further illustrated. The nidus is embedded in the cord parenchyma and extrapial space (top image). After resecting the extrapial portion via epipial dissection, the remaining parenchymal component is devascularized and obliterated (bottom image).
Figure 8: A final view of the operative field with the residual nidus within the cord after subtotal AVM removal by the pial resection technique.
With the development of the pial resection technique and recognition of its efficacy, myelotomies are now reserved for draining an intramedullary hematoma or an associated syrinx cavity. When complete resection is deemed feasible, longitudinal polar myelotomies over the dorsal midline, the dorsal root entry zone, and lateral or anterolateral midline may be done to access the nidus. Circumferential dissection by means of sharp preparation along the gliotic plane permits the lesion to be peeled away from the cord parenchyma, while additional deep feeding vessels can be ligated.
Feeding artery aneurysms and venous varices may be targeted for resection. They are extremely fragile and friable, even when partially thrombosed, so bipolar coagulation with broad tips is necessary to shrink these lesions before dissecting them from the cord surface.
Confirmation of surgical obliteration is routinely conducted via ICG or fluorescein fluorescent angiography. Formal spinal intraoperative angiography may be used with select complex lesions.
Further general principles regarding AVM resection can be found in the Nuances in AVM Resection chapter.
Closure
The dura is closed with running sutures in a watertight manner. Duraplasty is necessary to repair the resultant dural defect if the dural edges are not readily amenable to approximation.
Postoperative Considerations
The patient is observed in the intensive care unit overnight for frequent neurologic evaluations as well as pain and blood pressure control. Steroids are weaned slowly postoperatively.
The patient should remain lying flat for 24 to 48 hours postoperatively with the head of the bed gradually elevated.
Immediate postoperative formal spinal angiography is necessary to document lesional obliteration. Long-term follow-up imaging includes an angiogram 1, 3, 5, and 10 years after the operation. Symptomatic tethered spinal cord can manifest in delayed fashion and present with paresthesias. A detethering procedure is effective in these cases.
Pearls and Pitfalls
- Spinal AVMs are rare vascular malformations characterized by an intraparenchymal nidus.
- After studying the vascular anatomy of the malformation in detail, the pial resection technique can be considered as a relatively low-risk operative strategy for select lesions with predominantly exophytic components.
Contributor: Marcus André Acioly, MD, PhD
References
Black P. Spinal vascular malformations: an historical perspective. Neurosurg Focus. 2006;21:E11.
Dumont AS, Oldfield EH. Spinal vascular malformations (Chapter 397), in Richard Winn H (ed): Youmans Neurological Surgery, Vol 3, 6th Ed, Philadelphia: Elsevier Saunders, 2011, 4167-4202.
Flores BC, Klinger DR, White JA, Batjer HH. Spinal vascular malformations: treatment strategies and outcome. Neurosurg Rev. 2016 (in press).
Gross BA, Du R. Spinal glomus (type II) arteriovenous malformations: a pooled analysis of hemorrhage risk and results of intervention. Neurosurgery. 2013;72:25-32; discussion 32.
Kim LJ, Spetzler RF. Classification and surgical management of spinal arteriovenous lesions: arteriovenous fistulae and arteriovenous malformations. Neurosurgery. 2006;59(5 Suppl 3):S195-201;discussion S3-13.
Rangel-Castilla L, Spetzler RF. Microsurgical resection of cervical spinal arteriovenous malformation: the pial resection technique. Neurosurg Focus. 2014;37 Suppl 2:Video 12.
Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(2 Suppl):145-56.
Velat GJ, Chang SW, Abla AA, Albuquerque FC, McDougall CG, Spetzler RF. Microsurgical management of glomus spinal arteriovenous malformations: pial resection technique: clinical article. J Neurosurg Spine. 2012;16:523-31.
Weiss N, Bederson J, Post K. Management of spinal cord tumors and arteriovenous malformations (Chapter 188), in Quinones-Hinojosa A (ed): Schmidek & Sweet Operative Neurosurgical Techniques, Vol 2, 6th Ed, Philadelphia: Elsevier Saunders, 2012, 2135-2151.
Please login to post a comment.